Gas Laws
Gas Laws: Overview
This topic explores gas laws. It briefs on Boyle's, Charles's law and Gay-Lussac's law of gaseous volumes. It establishes the relation between pressure, volume, and temperature via these laws.
Important Questions on Gas Laws
Which one of these is not a correct example of Charles' Law?
Why does the spray bottle have the indication to keep them away from the sun and high temperature?
Graph between temperature and volume of the gas for Charles' Law is a straight line.
Which one of the following graphs shows the relation between pressure and volume taken as for Boyle's Law?
For Boyle's Law the graph for pressure and volume taken as is a straight line.
A certain mass of gas is taken in a container. At temperature being constant when the container is pressed its volume decreases. The true statement about this phenomenon is:
Boyle's Law states the relationship between _____ and volume of the gases.
Boyle's law states that the volume of an ideal gas is directly proportional to the absolute temperature at constant pressure.
Boyle's law is valid for
(i) pV = constant
(ii)
(iii) 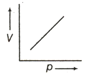
Which of the following is the correct option ?
According to the Boyle's law, as the pressure increases volume
Write important characteristics of a gas based upon kinetic theory of gases.
“At constant temperature, the pressure of a fixed amount of gas varies inversely with its volume”. This law is known as . Here, refers to:
Which of the expression represent the Boyle's law?
